FDA approves first at-home coronavirus test
Tuesday, April 21, 2020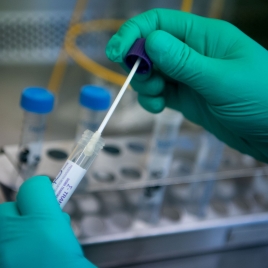
The Food and Drug Administration announced Tuesday that it approved the first authorized at-home coronavirus test. The test will be made available first to health care workers and first responders, and is expected to go on sale for consumers in most states within weeks.
The test, called the Pixel, is a nasal swab kit developed and sold by LabCorp. Patients will collect their own samples using a special sterile swab provided in the kit and then send it in to one of the company's labs for analysis.
The FDA said it granted the company emergency approval to get the tests out sooner.
"With this action, there is now a convenient and reliable option for patient sample collection from the comfort and safety of their home," FDA Commissioner Stephen Hahn said in a statement.
The test kit costs $119, and potential customers must complete a survey about their eligibility for testing before receiving one. The test's website says it will not be available in New York, New Jersey, Maryland and Rhode Island. New York and New Jersey are the two states with the highest number of coronavirus cases and deaths. CBS News has reached out to the company for more information.
LabCorp said the tests will initially be available to workers who may have been exposed to COVID-19 or may be symptomatic, and then the tests will go on sale for the public "in the coming weeks."
Health officials have warned consumers to avoid any non-approved home tests they may have seen promoted on social media, and the FDA repeated that warning in its statement Monday.
https://www.cbsnews.com/news/fda-approves-first-at-home-coronavirus-test/?fbclid=IwAR18SpiuQ93KEDH8Dg1cP0S4wT_YblNZB7jFO5ZXLX9Ssah8qaiKTBdPnew